Background
Anti-CD19 chimeric antigen receptor T-cells (CART) have been incorporated into the therapeutic landscape of B-acute lymphoblastic leukemia (B-ALL) and B-non-Hodgkin's lymphoma (B-NHL). The manufacturing process of commercially available autologous CART in patients relapsing after allogeneic hematopoietic cell transplant (allo-HCT) might include T-cells of donor origin. In this setting, there is limited data on graft-versus-host disease (GvHD) as an off-target effect in patients treated with CART after allo-HCT. We hereby report on a large, retrospective, EBMT registry-based study on GvHD in patients treated with CART therapy after allo-HCT.
Methods
Inclusion criteria were B-ALL and B-NHL adult and pediatric allo-HCT patients, treated with a first anti-CD19 CART (axicabtagene ciloleucel [axi-cel] and tisagenlecleucel [tisa-cel]) from 2018 to August 2022. The primary study endpoints were the cumulative incidences (CI) of new acute GvHD (aGvHD) and chronic GvHD (cGvHD). Secondary endpoints were the 1-year GvHD relapse-free survival (GRFS), non-relapse mortality (NRM), and overall survival (OS). Overall data was analyzed in a descriptive manner.
Results
A total of 257 allo-HCT patients treated with anti-CD19 CART were included. One hundred seventy-two patients (66.9%) were ≥18 years old. Tisa-cel was the therapy of choice in 184 patients (71.6%), whereas axi-cel was used in 73 patients (28.4%). More than half of the cohort (57.6%) underwent allo-HCT from unrelated donors. Notably, 109 patients (46.6%) and 38 patients (15%) had previously develop aGvHD and cGvHD between allo-HCT and CART infusion. Table 1 describes data on baseline patient, allo-HCT and CART characteristics of the whole cohort and of patients developing aGvHD and cGvHD.
In total, 3 patients developed new aGvHD and 6 patients developed new cGvHD after CART therapy. The 100-day CI of new aGvHD was 1.6% (95% CI, 0.4-4.2) and the 12-month CI of new cGvHD was 2.8% (95% CI, 1.1-5.7). No GvHD was observed in the pediatric cohort. The median time from allo-HCT to CART infusion was 15.8 months (range, 3.8-220.3) and the median times from CART to the development of aGvHD and cGvHD were respectively 44 days (range, 8-81) and 144 days (range, 5-182).
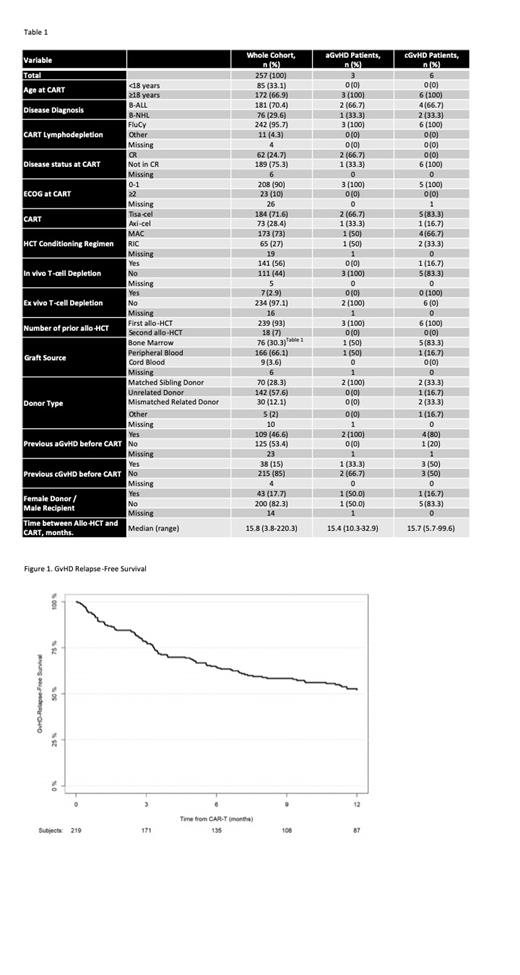
The 1-year GRFS and NRM were 52.1% (95% CI 45.6-59.4) and 4.7% (95% CI 2.5-8.1) respectively (Figure 1). With a median follow up of 18.8 months (95% CI, 16.2-23.7), the 1-year OS was 76.8% (95% CI 71.5-82.4). The most frequent cause of death was disease-related in 56 patients (74.6%).
Conclusion
Together, in this large cohort including adult and pediatric allo-HCT patients treated with tisa-cel and axi-cel after allo-HCT, these data show that the incidence of both aGvHD and cGvHD is very low. Of note, GvHD was not observed in the pediatric cohort. Furthermore, no excessive NRM was observed compared to previously published methods of treating relapse post allo-HCT, being disease progression a major hurdle to optimal outcomes in this cohort of patients.
Disclosures
Orti:JAZZ: Honoraria; Pfizer: Consultancy, Honoraria; Novartis: Honoraria; BMS: Honoraria; Incyte: Consultancy, Honoraria, Research Funding. Koenecke:Sanofi-Aventis: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Kite/Gilead: Consultancy; Janssen: Consultancy, Speakers Bureau; Medigene: Consultancy; Amgen: Consultancy; Glaxo Smith Kline: Consultancy; Miltenyi Biotec: Consultancy; Novartis: Consultancy, Speakers Bureau; Pierre Fabre: Consultancy; BMS: Consultancy; Pfizer: Consultancy. O'Reilly:Kite-Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Conference support; Novartis: Honoraria, Other: Conference support; Janssen: Honoraria; Autolus: Membership on an entity's Board of Directors or advisory committees. Balduzzi:Neovii: Speakers Bureau; Medac: Speakers Bureau; Agmen: Speakers Bureau; Novartis: Speakers Bureau. Besley:Kite, Novartis, Janssen and Takeda: Honoraria. Wynn:Orchard Therapeutics: Patents & Royalties: Milestone payments MPSIIIA clinical trial, Research Funding; AVRO BIO: Consultancy, Patents & Royalties: Milestone payments MPSII clinical trial, Research Funding. Bader:Neovii: Research Funding; BMS: Research Funding; Novartis: Consultancy, Research Funding; Medac: Consultancy, Patents & Royalties: medac, Research Funding. Mielke:Immunicum/Mendes, Miltenyi: Other: Participation on a Data Safety Monitoring Board or Advisory Board; SWECARNET: Other: Founder/Leadership (via my institution) ; ScientifyResearch: Other: Founder (spouse) ; Celgene/BMS, Novartis, Janssen, Gilead/KITE, JSMO, Pfizer: Speakers Bureau. Amrolia:Autolus PLC: Patents & Royalties: via UCL Business. Yakoub-Agha:Janssen: Honoraria; Bristol-Myers Squibb: Honoraria; Novartis: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support. Kröger:Neovii Biotech: Honoraria, Research Funding; MSD: Honoraria; Jazz: Honoraria; Kite/Gilead: Honoraria; Novartis: Honoraria, Research Funding; Pfizer: Honoraria; Riemser: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Takeda: Consultancy; Sanofi: Honoraria. Kwon:Jazz: Speakers Bureau; Pfizer: Speakers Bureau; Kite-Gilead: Consultancy, Speakers Bureau. Schoemans:Gilead: Other: Travel Support; Novartis: Honoraria, Research Funding; Janssen: Honoraria; Sanofi: Consultancy; Pfizer: Other: Travel Support. Penack:Gilead, Jazz, MSD, Novartis, Pfizer and Therakos: Honoraria, Other: Travel support; Incyte and Priothera: Research Funding; Equillium Bio, Jazz, Gilead, Novartis, MSD, Omeros, Priothera, Sanofi, Shionogi and SOBI: Membership on an entity's Board of Directors or advisory committees. Peric:Sanofi: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal